How to Effectively Read a Pka Table
A Key Skill – How To Use A pKa Table
Today we'll talk virtually an incredibly important skill that might take some time to grasp but pays tremendous dividends. We'll go through the exact details of how to apply a pKa table. [Background for pKa – read this mail service ] Understanding the proper use of a pKa table will give you the ability to recognize which acid-base of operations reactions volition happen and which will not. This will come a lot as you progress through Org 1 and Org 2. It might be helpful to get back and review some of the factors that touch acerbity that were talked well-nigh earlier.
Table of Contents
- A Sample Acid-Base Question
- The Cardinal Dominion Of Acrid-Base Reactions: In A Favorable Acid-Base Reaction, A Stronger Acid Plus A Stronger Base Produces A Weaker Acid and A Weaker Base of operations
- Use The "The Weaker The Acid, The Stronger The Conjugate Base" Principle To Obtain The Strengths Of Bases From A pKa Tabular array
- Draw Out The 4 Components Of The Acid-Base of operations Reaction (Acid, Base, Conjugate Acid, Conjugate Base of operations) And Evaluate Whether The Reaction Follows The Primal Dominion
- Dealing With Compounds That Are Similar, Only Not On The pKa Table
one. A Sample Question
Let'south say you're given the post-obit question:
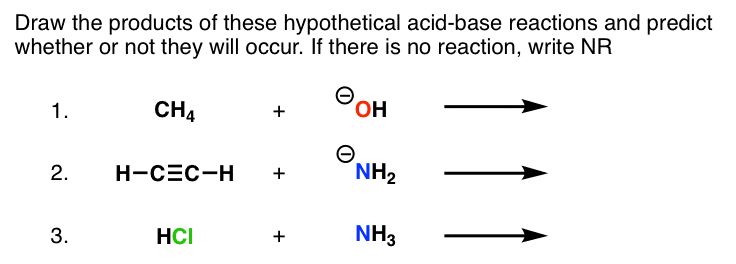
Past acidity, we're talking about Bronsted acerbity here – in other words, the ability to donate a proton.
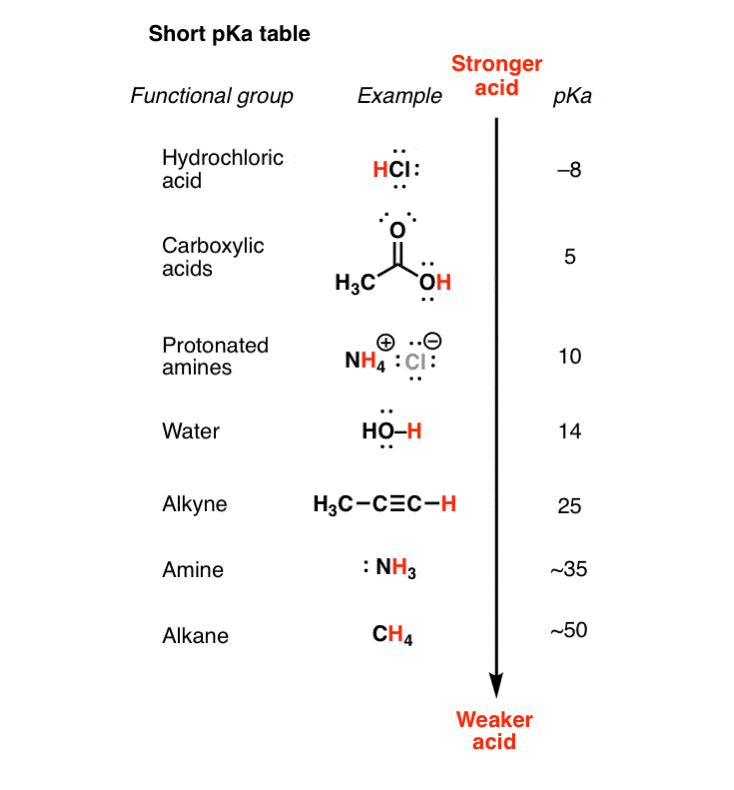
Let'southward say nosotros're given a pKa table with the post-obit values.
2. The Central Rule Of Acrid-Base Reactions: Stronger Acid Plus A Stronger Base Produces A Weaker Acid and A Weaker Base
Where practise we showtime with this problem?
- Remember that a pKa table ranks molecules in order of their acidity, from strongly acidic (east.g. HCl with pKa of –8) to weakly acidic (e.g. methane, pKa of ~50).
- What determines whether or not an acrid-base reaction will happen in the beginning place? Nosotros employ the post-obit principle to acid-base reactions: A stronger acid will tend to react with a stronger base to produce a weaker acid and a weaker base.
- It's piece of cake enough to employ a pKa table to determine acid force – we tin run across at a glance that H2O (pKa of xv) is a stronger acid than NH3 (pKa of 38). The question is, how do we determine base strength?
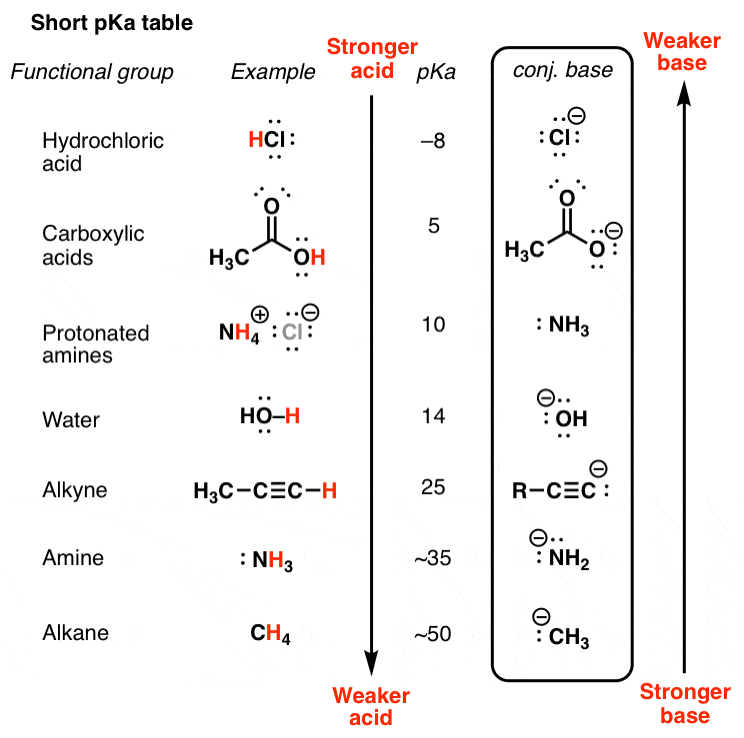
Here'southward how nosotros practice it. Draw out the conjugate bases of the acids on your pka table by removing a proton.
East.g. NH3 –> NH2(-) or CH4 –> CH3 (-).
3. Use The "The Weaker The Acrid, The Stronger The Conjugate Base" Principle To Obtain The Strengths Of Bases From A pKa Tabular array
Here's the central principle: The order of base of operations strength is the changed of acid strength. The weaker the acrid, the stronger the conjugate base. Using this principle, you tin also use the pKa table to give you the strengths of bases. I call this the inverse pKa table.
Here's a pKa table with the conjugate bases included:
four. Draw Out The 4 Components Of The Acid-Base Reaction (Acid, Base, Conjugate Acid, Conjugate Base) And Evaluate Whether The Reaction Follows The Fundamental Rule
4 . Here's how nosotros apply this noesis to the problem.
Observe the acid on the pKa table. Find the base of operations on the inverse pKa table. Do the acid base reaction – that is, add a proton to the base and remove a proton from the acid.
5. Evaluate: Is the new acid stronger or weaker? Is the new base stronger or weaker?
6. Examples.
Example A : Nosotros have CH4 and HO(-) We can find CH4 on the pKa tabular array – it has a pKa of l. Hydroxide ion, HO(-) is not on the left side of the pKa tabular array, but it is on the "inverse" pKa tabular array – it is the conjugate base of water, HtwoO. So CHiv is the acid and HO(-) is the base in this reaction.
Doing the proposed acid base reaction, nosotros transfer a proton from CH4 to HO (-). The products of this reaction would therefore be CH3(-) and h2o.
Now nosotros ask the question – how do these compare in strength to our starting acids and bases? H2o has a pKa of ~15, and CH4 has a pKa of 50. Our product is a stronger acid. From the inverse pKa table, we also note that CH3(-) is a stronger base than HO(–). Our production is a stronger base of operations.
Verdict – the reaction won't happen. We demand to go to a weaker acrid-base of operations pair (see #2, to a higher place). Then nosotros write "NR".
Hither's another case.
Example B – Have HCΞCH and NHii(–). HCΞCH has a pKa of 25; on the other hand, NHii(–) is on the conjugate base table. Drawing out the products of the acid base reaction will requite united states of america NHiii (weaker acid than HCΞCH) and HCΞC(–) (weaker base of operations than NHtwo(–). This reaction will go.
Instance C : Accept NHiii and HCl. This time, we can find both HCl and NH3 on the pKa table. But HCl has a pKa of (–8) and NH3 has a pKa of 38. HCl will clearly deed as an acid here, and NH3 will act every bit a base of operations.
We tin write out our acid base reaction: HCl + NHthree → NH4(+) Cl(–)
Our acidic production, NH4, has a pKa of nine. Our product is a weaker acid than HCl. Our bones production, Cl(-) ranks below NH3 on our changed pKa scale. Our product is a weaker base than NH3. Determination: this reaction is also a get. And, indeed, if yous find yourself in a freezing hut with just a bottle of concentrated HCl and aqueous ammonia to keep you company, adding them together will definitely warm upwards your mean solar day. This is about the merely situation in which I would recommend this.
5. Dealing With Compounds That Are Similar, Merely Not On The pKa Table
Q. How practice y'all deal with a compound that is like just not on the table? Have hexane, for instance. Fifty-fifty though it is not technically on the list, its behavior is similar enough to methane – they're both alkanes, subsequently all – that we make the assumption that the pKa's are roughly the same. Similarly, an amine like trimethylamine [N(CH3)3] will have similar behavior to NH3 in the reaction with HCl.
One final point on the large-picture type view. Notation the pattern. The conjugate base of methane (HthreeC(–) ) is potent plenty to deprotonate anything below it on the pKa table (that is, pretty much everything). Methyllithium, CH3Li, is an incredibly potent base of operations. Conversely, acetylide ion, HCC(–) is strong enough to effectively deprotonate any acid with a pKa under ~25, and acetate ion (CH3COO(–)) is weaker still, only able to deprotonate any acid with a pKa lower than v.
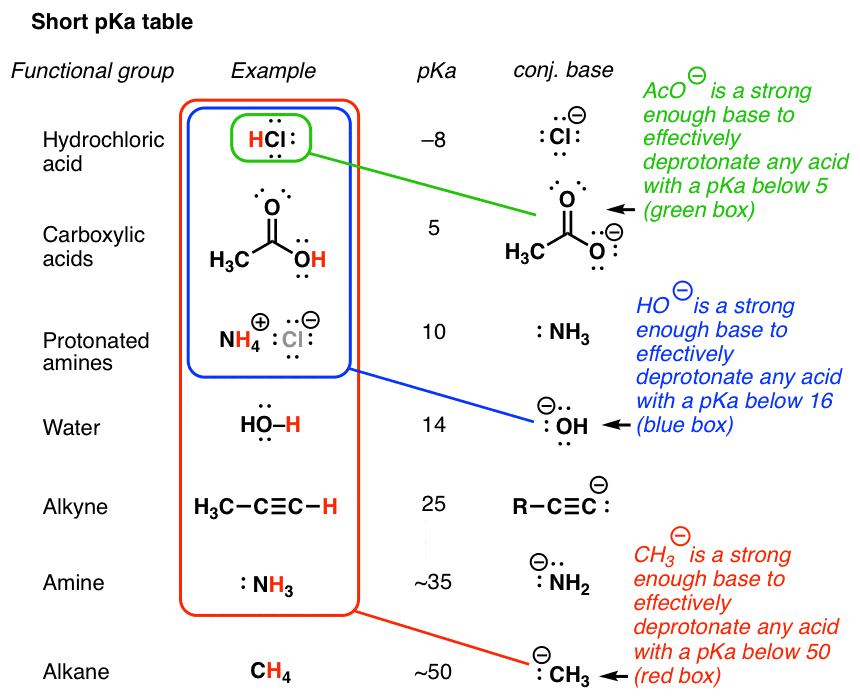
That's why I compare a strong base like methyl lithium to a purple flush in poker – it volition essentially defeat any paw (acid) it encounters.
Next Post:A Handy Rule of Thumb for Acrid Base Reactions
Source: https://www.masterorganicchemistry.com/2010/09/29/how-to-use-a-pka-table/
0 Response to "How to Effectively Read a Pka Table"
Post a Comment